Non-Hodgkin’s Lymphoma Market Forecast to Surge with Growing Adoption of Targeted and Cell-Based Therapies | DelveInsight
The non-Hodgkin’s lymphoma market size is expected to increase during the forecast period (2025–2034) owing to increasing incidence, advancements in targeted and precision therapies, the launch of potential emerging therapies, improving CAR-T manufacturing challenges to increase scalability, and increased awareness and early detection efforts.
New York, USA, July 24, 2025 (GLOBE NEWSWIRE) -- Non-Hodgkin’s Lymphoma Market Forecast to Surge with Growing Adoption of Targeted and Cell-Based Therapies | DelveInsight
The non-Hodgkin’s lymphoma market size is expected to increase during the forecast period (2025–2034) owing to increasing incidence, advancements in targeted and precision therapies, the launch of potential emerging therapies, improving CAR-T manufacturing challenges to increase scalability, and increased awareness and early detection efforts.
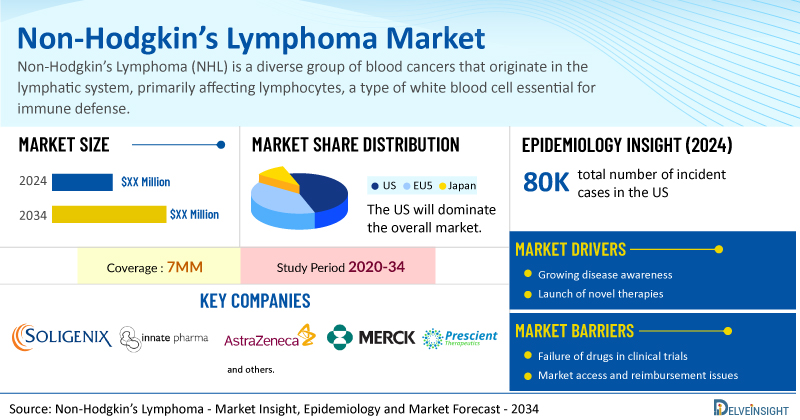
DelveInsight’s Non-Hodgkin’s lymphoma Market Insights report includes a comprehensive understanding of current treatment practices, emerging non-Hodgkin’s lymphoma drugs, market share of individual therapies, and current and forecasted non-Hodgkin’s lymphoma market size from 2020 to 2034, segmented into 7MM [the United States, the EU4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan].
Key Takeaways from the Non-Hodgkin’s Lymphoma Market Report
- According to DelveInsight’s analysis, the total non-Hodgkin’s lymphoma market size is expected to grow positively by 2034.
- The United States accounts for the largest market size of Non-Hodgkin’s Lymphoma, in comparison to EU4 (Germany, Italy, France, and Spain) and the UK, and Japan.
- In the US, the total number of incident cases of NHL in 2024 were ~80K. In 2024, B-cell NHL accounted for approximately 85% of all NHL cases, while T-cell and Natural Killer (NK)-cell NHL comprised the remaining 15%.
- Prominent companies, including Soligenix, Innate Pharma, Nurix Therapeutics, AstraZeneca, Merck, Prescient Therapeutics, and others, are actively working on innovative non-Hodgkin’s lymphoma drugs.
- Some of the key non-Hodgkin’s lymphoma therapies in the pipeline include HyBryte, Lacutamab, Zelebrudomide (NX-2127) and Bexobrutideg (NX-5948), CALQUENCE (acalabrutinib), Zilovertamab Vedotin (MK-2140), PTX-100, and others. These novel non-Hodgkin’s lymphoma therapies are anticipated to enter the non-Hodgkin’s lymphoma market in the forecast period and are expected to change the market.
- In June 2025, Nurix Therapeutics announced the positive clinical data from the Company’s ongoing NX-5948-301 study, a Phase Ia/b clinical trial of bexobrutideg (NX-5948) in patients with R/R B-cell malignancies.
- In April 2025, Roche announced that the European Commission (EC) had approved COLUMVI in combination with gemcitabine and oxaliplatin (GemOx) for the treatment of adult patients with relapsed or refractory (R/R) DLBCL not otherwise specified who are ineligible for autologous stem cell transplant (ASCT).
Discover which non-Hodgkin’s lymphoma medications are expected to grab the market share @ Non-Hodgkin’s Lymphoma Market Report
Non-Hodgkin’s Lymphoma Overview
Non-Hodgkin’s Lymphoma (NHL) is a diverse group of blood cancers that originate in the lymphatic system, primarily affecting lymphocytes, a type of white blood cell essential for immune defense. Unlike Hodgkin’s lymphoma, NHL lacks the characteristic Reed-Sternberg cells. The causes of NHL are multifactorial, involving a combination of genetic, environmental, and immune-related factors. Risk factors include a weakened immune system, certain infections, autoimmune diseases, exposure to chemicals such as pesticides, and a family history of lymphoma.
Symptoms of NHL can vary widely depending on the disease subtype and progression, but commonly include painless swelling of lymph nodes, especially in the neck, armpits, or groin. Patients may also experience systemic symptoms such as fever, night sweats, unexplained weight loss (known as "B symptoms"), fatigue, and persistent infections. In some cases, there may be abdominal pain or swelling, chest pain, coughing, or difficulty breathing if lymph nodes in those areas are affected.
Diagnosis of NHL typically involves a combination of clinical evaluation and diagnostic tests. A biopsy of the enlarged lymph node or affected tissue is crucial for confirming the presence of lymphoma cells and determining the specific NHL subtype. Additional tests, such as blood tests, imaging studies like CT scans, PET scans, and MRI, help assess the extent and stage of the disease. Bone marrow aspiration and biopsy may be performed to check for bone marrow involvement. Molecular and genetic testing further guides treatment decisions by identifying specific mutations or markers associated with the lymphoma.
Non-Hodgkin’s Lymphoma Epidemiology Segmentation
The non-Hodgkin’s lymphoma epidemiology section provides insights into the historical and current non-Hodgkin’s lymphoma patient pool and forecasted trends for the 7MM. It helps recognize the causes of current and forecasted patient trends by exploring numerous studies and views of key opinion leaders.
The non-Hodgkin’s lymphoma market report proffers epidemiological analysis for the study period 2020–2034 in the 7MM, segmented into:
- Total Incident Cases of NHL
- Type-specific Incident Cases of NHL
- Subtype-specific Incident Cases of NHL
- Line-wise Treated Cases of NHL
Download the report to understand which factors are driving non-Hodgkin’s lymphoma epidemiology trends @ Non-Hodgkin’s Lymphoma Treatment Algorithm
Non-Hodgkin’s Lymphoma Treatment Market
Management of NHL generally includes a range of treatment strategies such as combination chemotherapy, localized radiotherapy, stem cell transplants, steroid therapy, targeted agents, immunotherapies, and CAR T-cell therapies, tailored according to the disease subtype and stage.
Several drugs have been approved for NHL treatment, including BELEODAQ (Acrotech Biopharma), POTELIGEO (Kyowa Hakko Kirin), ADCETRIS (Pfizer and Takeda), LYMPHIR (Citius Pharmaceuticals), EZHARMIA (Daiichi Sankyo), POLIVY (Genentech/Roche), YESCARTA (Kite Pharma/Gilead Sciences), EPKINLY (Genmab/AbbVie), KYMRIAH (Novartis), TAZVERIK (Epizyme/Ipsen), COLUMVI (Genentech), MOUNJUVI (MorphoSys/Incyte), LUNSUMIO (Genentech/Biogen), KEYTRUDA (Merck), and others.
Autologous CAR T-cell therapies such as YESCARTA, BREYANZI, KYMRIAH, and TECARTUS have demonstrated significant clinical benefits but are hindered by high treatment costs, complex production processes, manufacturing delays, and the risks of toxicity and potential production failure.
Currently, no molecular or biological markers exist to predict disease progression in early-stage CTCL or to identify advanced-stage patients likely to experience prolonged survival.
While follicular lymphoma typically follows a slow disease course, early relapse or transformation can significantly worsen outcomes, and current prognostic models like the Follicular Lymphoma International Prognostic Index (FLIPI), based on clinical parameters, offer limited predictive precision.
Learn more about the non-Hodgkin’s lymphoma treatment options @ Non-Hodgkin’s Lymphoma Treatment Guidelines
Non-Hodgkin’s Lymphoma Emerging Drugs and Companies
Some of the products in the pipeline include HyBryte (Soligenix), Lacutamab (Innate Pharma), Zelebrudomide (NX-2127) and Bexobrutideg (NX-5948) (Nurix Therapeutics), CALQUENCE (acalabrutinib) (AstraZeneca), Zilovertamab Vedotin (MK-2140) (Merck), PTX-100 (Prescient Therapeutics), and others.
HyBryte is a topical formulation containing hypericin, one of the most light-sensitive substances identified. The ointment is applied in a thin layer to CTCL lesions and left covered for 18 to 24 hours, after which the area is exposed to focused visible light. Upon light activation, hypericin triggers the destruction of malignant T cells within the lesion. HyBryte has been granted Orphan Drug and Fast Track designations by the FDA and has also received orphan status from the European Medicines Agency (EMA).
Soligenix shared new data from supporting clinical trials evaluating HyBryte for CTCL treatment at the United States Cutaneous Lymphoma Consortium (USCLC) Workshop on March 6, 2025, and the American Academy of Dermatology (AAD) Annual Meeting held from March 7 to 11, 2025. In an April 2024 press release, Soligenix stated it expects to release top-line results from its confirmatory Phase III FLASH2 trial, an 18-week study conducted in the U.S. and Europe, in the second half of 2026.
Zilovertamab vedotin is an experimental ADC targeting the Receptor Tyrosine Kinase-like Orphan Receptor 1 (ROR1). The waveLINE clinical program features several trials, including a Phase II/III study in relapsed/refractory diffuse large B-cell lymphoma (DLBCL) (waveLINE-003, NCT05139017), a Phase III trial in newly diagnosed DLBCL patients (waveLINE-010), a Phase II study in aggressive and indolent B-cell malignancies (NCT05458297), and a Phase I trial in hematological cancers (NCT03833180).
In May 2025, Merck announced findings from the dose confirmation segment of the Phase II/III waveLINE-003 trial, which evaluated zilovertamab vedotin combined with rituximab and gemcitabine-oxaliplatin (R-GemOx) in patients with relapsed/refractory DLBCL.
Lacutamab is a first-in-class humanized antibody against KIR3DL2 that induces cytotoxic activity. It is under investigation in an open-label, multicohort Phase II trial for CTCL, as well as in a separate Phase II study in PTCL.
According to a March 2025 press release, updated long-term results from the TELLOMAK Phase II trial in Sézary syndrome and mycosis fungoides will be presented at the 2025 ASCO Annual Meeting on June 2 in Chicago, Illinois. Innate Pharma’s May 2025 annual updates indicated that a Phase III trial of lacutamab is planned, with a submission for accelerated approval anticipated in 2027.
The anticipated launch of these emerging non-Hodgkin’s lymphoma therapies are poised to transform the non-Hodgkin’s lymphoma market landscape in the coming years. As these cutting-edge non-Hodgkin’s lymphoma therapies continue to mature and gain regulatory approval, they are expected to reshape the non-Hodgkin’s lymphoma market landscape, offering new standards of care and unlocking opportunities for medical innovation and economic growth.
To know more about new treatment for Non-Hodgkin’s Lymphoma, visit @ Non-Hodgkin’s Lymphoma Management
Non-Hodgkin’s Lymphoma Market Dynamics
The non-Hodgkin’s lymphoma market dynamics are anticipated to change in the coming years. The management of NHL involves multiple standard-of-care therapies, including R-CHOP, CAR T-cell therapies, monoclonal antibodies, and bispecific antibodies, guided by well-established protocols from NCCN, ESMO, and ASH, while the emergence of allogeneic CAR T-cell products offers greater accessibility by potentially reducing manufacturing delays and broadening treatment availability for patients requiring urgent care; in parallel, opportunities exist for companies to develop or expand therapies for R/R 2L transplant-eligible DLBCL patients, where Salvage Chemotherapy remains the mainstay alongside the recent introduction of YESCARTA and BREYANZI, and advancements in molecular techniques, such as flow cytometry, polymerase chain reaction, and high-throughput sequencing, are driving earlier diagnosis, improved prognostication, and the development of more effective, individualized therapies, including for patients with CTCL.
Furthermore, many potential therapies are being investigated for the treatment of non-Hodgkin’s lymphoma, and it is safe to predict that the treatment space will significantly impact the non-Hodgkin’s lymphoma market during the forecast period. Moreover, the anticipated introduction of emerging therapies with improved efficacy and a further improvement in the diagnosis rate is expected to drive the growth of the non-Hodgkin’s lymphoma market in the 7MM.
However, several factors may impede the growth of the non-Hodgkin’s lymphoma market. T-cell malignancies such as PTCL-NOS and ETP-ALL are aggressive and difficult to treat, with rapid progression, poor response to standard therapies, and frequent relapses despite initial remission, while the treatment landscape faces additional challenges including the limitations of targeted therapies in CTCL due to toxicity and resistance, manufacturing and scalability issues with CAR T-cell therapies in NHL where R-CHOP remains the gold standard, and increasing competitive and pricing pressures from the entry of rituximab biosimilars impacting original branded products.
Moreover, Non-Hodgkin’s Lymphoma treatment poses a significant economic burden and disrupts patients’ overall well-being and QOL. Furthermore, the non-Hodgkin’s lymphoma market growth may be offset by failures and discontinuation of emerging therapies, unaffordable pricing, market access and reimbursement issues, and a shortage of healthcare specialists. In addition, the undiagnosed, unreported cases and the unawareness about the disease may also impact the non-Hodgkin’s lymphoma market growth.
| Non-Hodgkin’s Lymphoma Report Metrics | Details |
| Study Period | 2020–2034 |
| Non-Hodgkin’s Lymphoma Report Coverage | 7MM [The United States, the EU-4 (Germany, France, Italy, and Spain), the United Kingdom, and Japan] |
| Key Non-Hodgkin’s Lymphoma Companies | Soligenix, Innate Pharma, Nurix Therapeutics, AstraZeneca, Merck, Prescient Therapeutics, Kite Pharma, Seagen, Takeda, Novartis, Daiichi Sankyo, Genmab, AbbVie, Genentech (a Member of Roche), Bristol Myers Squibb, and others |
| Key Non-Hodgkin’s Lymphoma Therapies | HyBryte, Lacutamab, Zelebrudomide (NX-2127) and Bexobrutideg (NX-5948), CALQUENCE (acalabrutinib), Zilovertamab Vedotin (MK-2140), PTX-100, YESCARTA, ADCETRIS, KYMRIAH, EZHARMIA, EPKINLY/TEPKINLY, COLUMVI, BREYANZI, and others |
Scope of the Non-Hodgkin’s Lymphoma Market Report
- Non-Hodgkin’s Lymphoma Therapeutic Assessment: Non-Hodgkin’s Lymphoma current marketed and emerging therapies
- Non-Hodgkin’s Lymphoma Market Dynamics: Conjoint Analysis of Emerging Non-Hodgkin’s Lymphoma Drugs
- Competitive Intelligence Analysis: SWOT analysis and Market entry strategies
- Unmet Needs, KOL’s views, Analyst’s views, Non-Hodgkin’s Lymphoma Market Access and Reimbursement
Discover more about non-Hodgkin’s lymphoma drugs in development @ Non-Hodgkin’s Lymphoma Clinical Trials
Table of Contents
| 1. | Non-Hodgkin’s Lymphoma Market Key Insights |
| 2. | Non-Hodgkin’s Lymphoma Market Report Introduction |
| 3. | Non-Hodgkin’s Lymphoma Market Overview at a Glance |
| 4. | Non-Hodgkin’s Lymphoma Market Executive Summary |
| 5. | Disease Background and Overview |
| 6. | Non-Hodgkin’s Lymphoma Treatment and Management |
| 7. | Non-Hodgkin’s Lymphoma Epidemiology and Patient Population |
| 8. | Patient Journey |
| 9. | Non-Hodgkin’s Lymphoma Marketed Drugs |
| 10. | Non-Hodgkin’s Lymphoma Emerging Drugs |
| 11. | Seven Major Non-Hodgkin’s Lymphoma Market Analysis |
| 12. | Non-Hodgkin’s Lymphoma Market Outlook |
| 13. | Potential of Current and Emerging Therapies |
| 14. | KOL Views |
| 15. | Unmet Needs |
| 16. | SWOT Analysis |
Related Reports
Non-Hodgkin’s Lymphoma Pipeline
Non-Hodgkin’s Lymphoma Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key NHL companies, including Bristol-Myers Squibb, Beijing Mabworks Biotech, CARGO Therapeutics, Guangzhou Lupeng Pharmaceutical, Ryvu Therapeutics, Dren Bio, ImmunityBio, Merck, EntreChem, Bantam Pharmaceutical, Vironexis Biotherapeutics, Excyte Biopharma, Owkin, AstraZeneca, ST Phi Therapeutics, NovImmune SA, among others.
Cutaneous T-cell Lymphoma Market
Cutaneous T-cell Lymphoma Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key CTCL companies, including Soligenix and Sterling Pharma Solutions, Prescient Therapeutics, Innate Pharma, Bristol-Myers Squibb, ONO Pharmaceutical, among others.
Follicular Lymphoma Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key follicular lymphoma Market companies, including Merck Sharp and Dohme, AstraZeneca, CRISPR Therapeutics, BeiGene, Nektar Therapeutics, NovalGen, Carna Biosciences, Allogene Therapeutics, Xynomic Pharmaceuticals, Bristol-Myers Squibb, Incyte Corporation, among others.
Multiple Myeloma Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key multiple myeloma companies, including Sanofi, Karyopharm Therapeutics, AbbVie, Takeda Pharmaceutical, Celgene, Bristol-Myers Squibb, RAPA Therapeutics, Pfizer, Array Biopharma, Cellectar Biosciences, BioLineRx, Celgene, Aduro Biotech, ExCellThera, Janssen Pharmaceutical, Precision BioSciences, Takeda, Glenmark (Ichnos Sciences SA), Poseida Therapeutics, Molecular Partners AG, Chipscreen Biosciences, AbbVie, Genentech (Roche), Janssen Biotech, Nanjing Legend Biotech, Merck Sharp & Dohme Corp., among others.
Diffuse Large B-cell Lymphoma Market
Diffuse Large B-cell Lymphoma Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key DLBCL companies, including Roche (Genentech), Biogen, Nektar Therapeutics, Merck, Allogene Therapeutics, Miltenyi Biomedicine, AstraZeneca, BioVaxys, ImmunoVaccine Technologies, Cellectar Biosciences, Galapagos, Novartis, Lyell, ImmPACT Bio, Pfizer, Kartos Therapeutics, 2seventy bio, Regeneron Pharmaceuticals, BeiGene, Ranok Therapeutics, Constellation Pharmaceuticals, Genmab, IDP Discovery Pharma S.L., Immunitas Therapeutics, Monte Rosa Therapeutics, SymBio Pharmaceuticals, AVM Biotechnology, Autolus Therapeutics, Kymera Therapeutics, Otsuka Pharmaceutical, Caribou Biosciences, Adicet Bio, Gilead Sciences, Xynomic Pharmaceuticals, Amgen, among others.
DelveInsight’s Pharma Competitive Intelligence Service: Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Pharmaceutical Consulting Services
Healthcare Conference Coverage
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences. It supports pharma companies by providing comprehensive end-to-end solutions to improve their performance. Get hassle-free access to all the healthcare and pharma market research reports through our subscription-based platform PharmDelve.
Connect with us on LinkedIn|Facebook|Twitter

Contact Us Shruti Thakur info@delveinsight.com +14699457679 www.delveinsight.com
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
